Design, test, and produce complex biological molecules in a modern LIMS
Before Gilead and Benchling started working together two years ago, the Biologic Development division was tracking and managing samples, experimental requests, and large data sets using a mixture of email, Excel, and on-premise electronic lab notebooks (ELN). While it was a serviceable solution, the team was hungry for greater visibility into their data so that they could derive more insights and improve productivity. The Biologic Development team began looking for an integrated solution that could serve their teams and workflows more holistically. They turned to Benchling’s R&D Cloud to configure a compliant, easy-to-use, adaptable, and connected sample management process.
Over the past several years, Benchling has focused on building a whole new approach to LIMS, answering the deep unmet need from many R&D teams for a better solution to manage samples and process-driven work serving biology’s unique needs. With the Benchling R&D Cloud, research, analytical, bioanalytical, and process development teams can work together with greater confidence to get breakthrough molecules to milestones faster for both non-GxP and GxP compliant workflows. Like Gilead, teams at CUE, Jounce, Editas, and Syngenta now have a shared solution across their teams to manage samples and processes.
Legacy LIMS no longer work
Scientists developing biomolecules find legacy LIMS to be painfully in adequate for four primary reasons:
Built for small molecules
LIMS were built for chemists to manage small molecule testing and production and therefore cannot sufficiently handle the complexities of large biomolecules.
Disconnected
Usually deployed into specific sites or for specific teams, LIMS typically house data within a standalone system that doesn’t connect intelligence or processes with other R&D solutions. This disconnected web of software hinders collaboration and fragments data.
Brittle
As processes inevitably evolve over time, teams find LIMS overwhelmingly slow and difficult to adjust given their rigidity. In addition, legacy LIMS are nearly impossible to update in time to support scientific advances as new insights and opportunities open up novel ways of processing biological modalities.
Onerous to maintain
IT leaders spend an inordinate amount of time maintaining these legacy systems, creating workarounds and patches for changing needs and disconnected data.
Benchling is biology’s modern LIMS
In order for products to get to market successfully and quickly, biologists need to work together easily across specialized teams. To support this, the Benchling R&D cloud provides LIMS capabilities that are:
Built for biology
Benchling was specifically built for the complexities and nuances involved in designing, testing, and producing cell and gene therapies, antibody drug conjugates, RNA therapeutics, and more.
Connected
Working within a shared digital solution means scientists in research, bioprocess and bioanalytical development can access relevant information to carry work forward with minimal friction. Collaboration is an integrated part of the workflow rather than done separately via email or in meetings. Because it is cloud-native, Benchling can be easily implemented for one team and then extended to additional teams across sites and roles as needed. All work completed across resources – including integrated instruments – generates data in a common location for shared access and holistic transparency. Downstream development teams have context of previous work done upstream so they never have to waste time re-running experiments or re-creating data sets.
Adaptable
As procedures evolve over time and the pace of scientific progress uncovers new opportunities, teams won’t get stuck writing a bunch of code to adjust. Benchling is adaptable to support process innovation over time. Development teams can take advantage of methods innovation while staying within GxP guardrails to keep things secure and compliant.
Modern
Benchling users share a powerful, simple, and unified cloud-native workspace. With regular release updates, users always have the most up-to-date capabilities available in the market. IT can focus more on strategic, advanced tech initiatives rather than always patching and repairing old, disconnected systems.
Our work at Gilead requires managing highly complex, multi-dimensional data and necessitates technology partners that can support the scale and complexity of the data created by high-throughput biology. We selected Benchling as a partner because it's built for biology. By capturing structured data in Benchling, our scientists are empowered to ask challenging questions and uncover new insights.
All-in-one experiment, sample, and process management
With Benchling R&D Cloud, R&D teams work within a modern LIMS to:
Optimize handoffs across scientists and teams
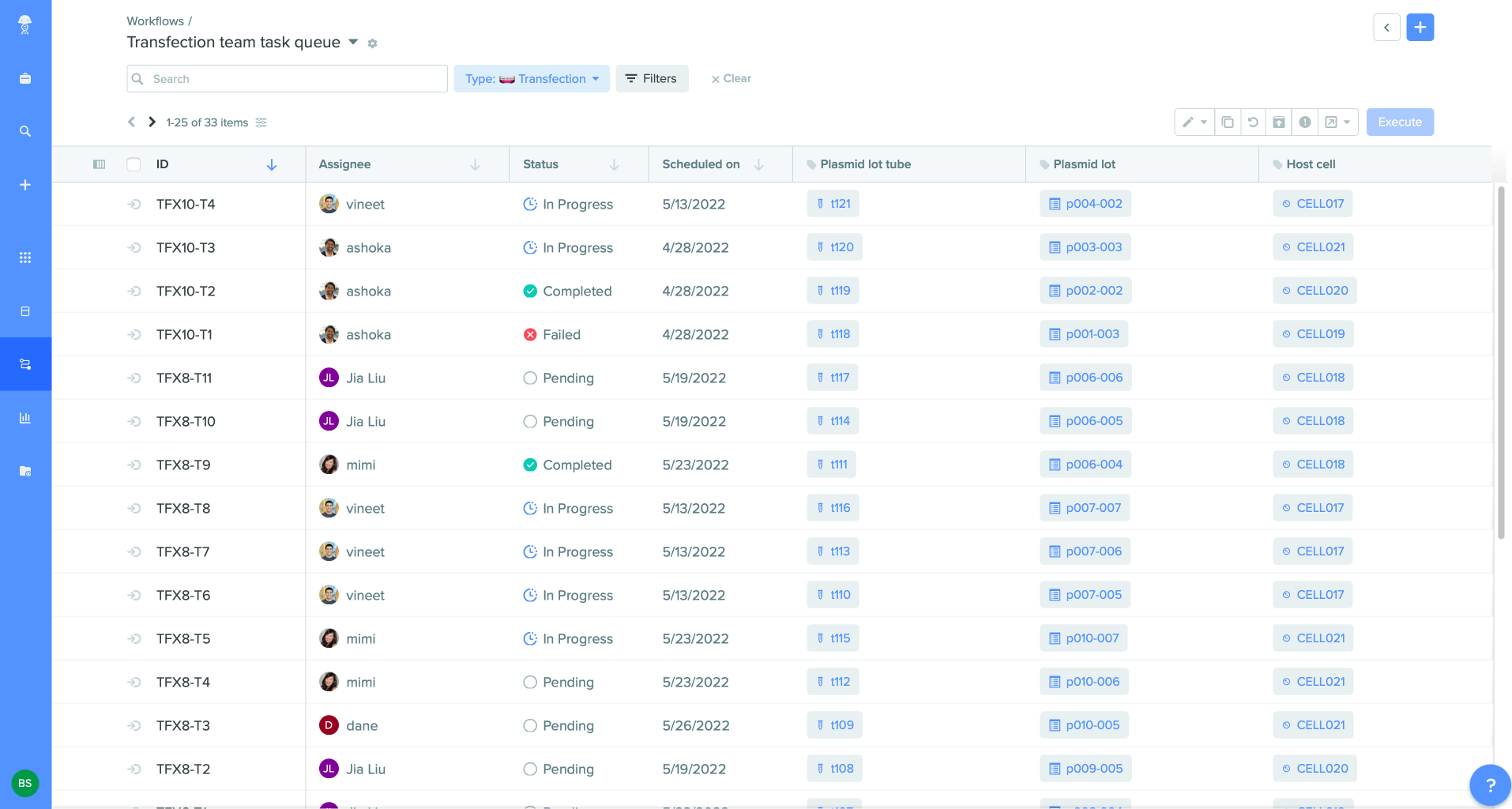
Research, Bioprocess, Bioanalytical, and Quality managers can establish workflows easily by quickly setting up procedure-specific templates and aligning activities among people, instruments, and teams. With the Workflows application, they can track progress and unblock bottlenecks by viewing task status across tasks with estimated time-to-completion and automatic notifications. All collaborators have context for completed work by leveraging existing data captured from prior tasks and assays, reducing redundant work.
Track samples throughout their lifecycle
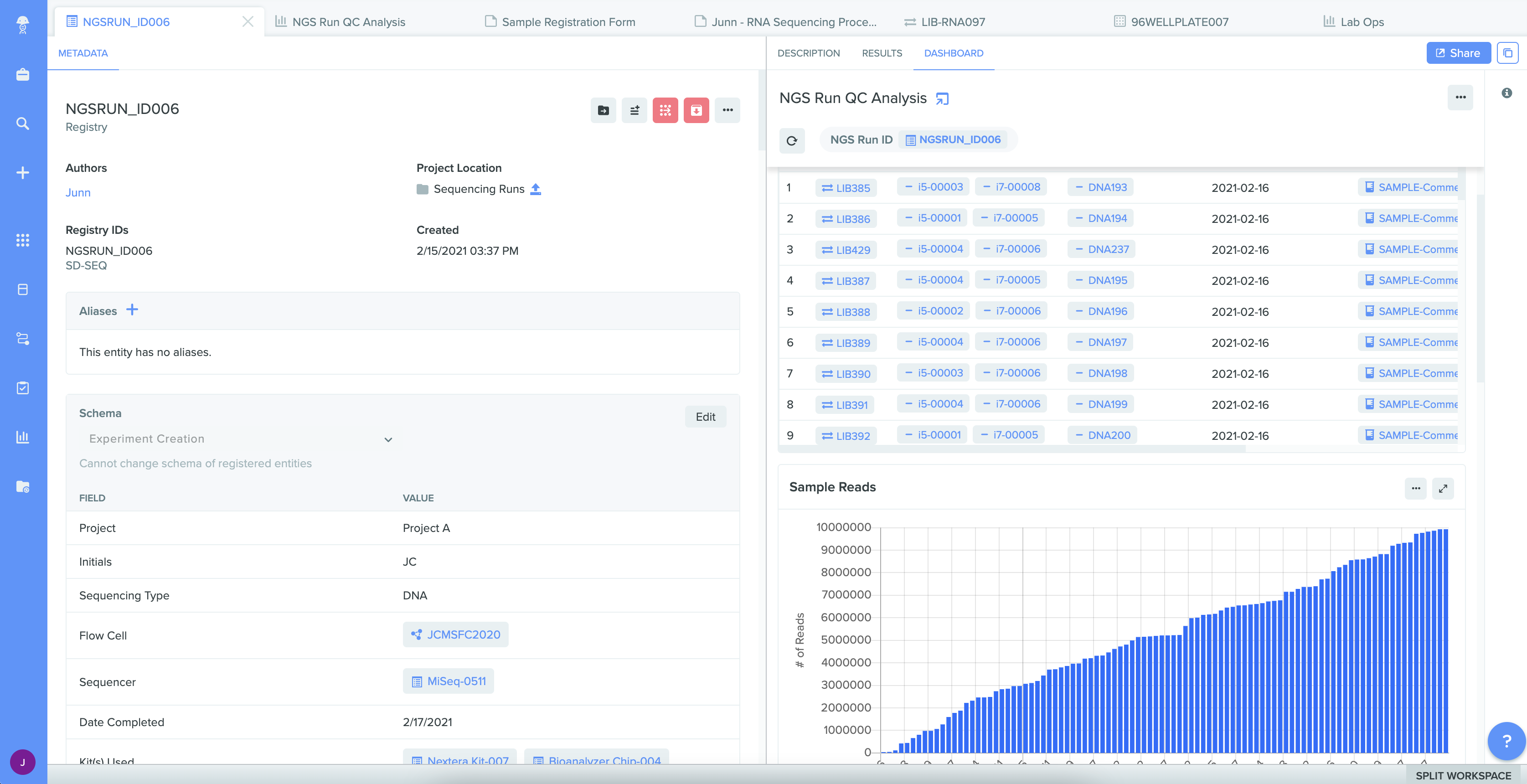
Everyone involved in sample management can drive complete sample history across the entire R&D journey. Sample lineage begins with easy registration – including integrated label printing and scanning – and efficient distribution for ongoing use. All sample testing procedures and results are captured in real-time. Lab and site managers can also increase inventory utilization by optimizing inventory consumption when quickly viewing and tracking lots in Benchling. And users with access can view all of this sample intelligence at any time.
Ensure compliance and data integrity
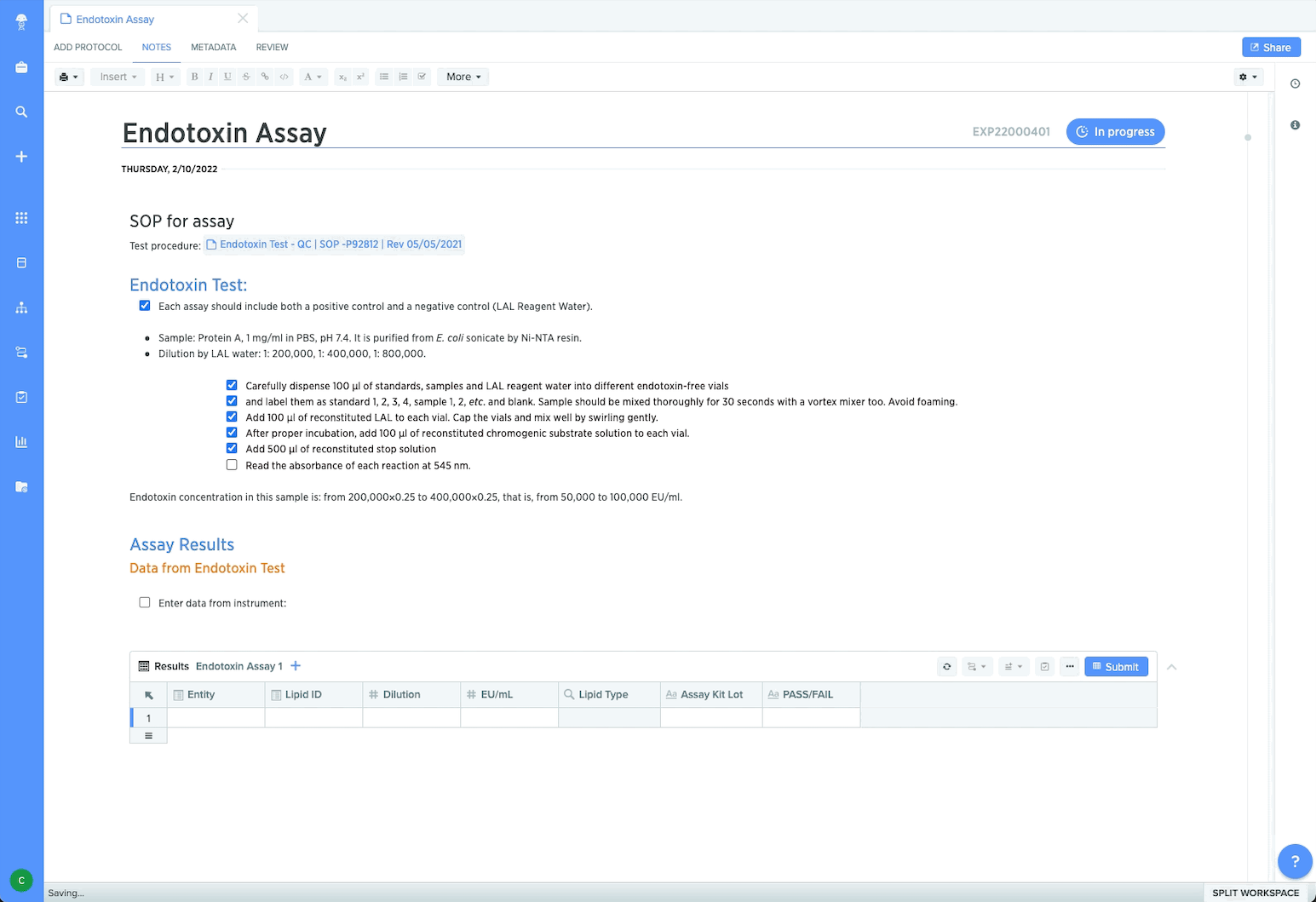
To manage locked-down procedures, process managers can control protocol execution using templates. This ensures data captured is consistent, high-quality, and aligned to SOPs. Managers and Quality can maintain reliable traceability since processes and sample work are captured within one system across teams and sites. By collaborating in Benchling’s GxP compliant environment, cross-functional teams can prepare for regulatory engagement with audit trails that include a detailed history of data modifications and user permissions controls. Plus customers working within Benchling’s Validated Cloud can select new feature updates confidently by taking advantage of vendor validation services.
Learn more
Learn more about how Benchling provides a modern, connected, and adaptable solution that redefines processes traditionally tracked in LIMS in this on-demand webinar.

